Pfizer and BioNTech announce FDA approval of their Covid-19 vaccine for young teens
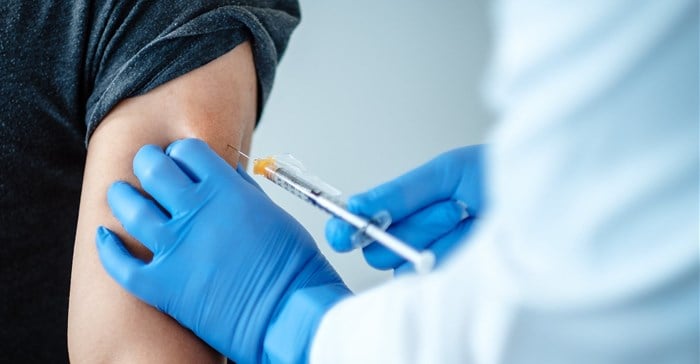
The vaccine was previously made available to this age group in the US under emergency use authorisation (EUA), and to date more than 9 million 12- to 15-year-old adolescents in the US. have completed a primary series.
The approval is based on data from a Phase 3 clinical trial of 2,260 participants 12 through 15 years of age.
A two-dose primary series of the vaccine (30-µg dose) elicited Sars-CoV-2–neutralising antibody geometric mean titers (GMTs) of 1,239.5, demonstrating strong immunogenicity in a subset of adolescents one month after the second dose.
This compared well to GMTs elicited by participants aged 16 to 25 years old (705.1 GMTs) in an earlier analysis.
Cominarty is now the only Covid-19 vaccine approved by the FDA as a two-dose primary series for individuals 12 years and older.
Pfizer and BioNTech have also submitted a sBLA to the US FDA to extend the approval of Cominarty to include booster doses for individuals ages 16 years and older, who are currently authorised under EUA.
Cominarty, which is based on BioNTech’s proprietary mRNA technology, was developed by both BioNTech and Pfizer.
BioNTech is the marketing authorisation holder in the United States, the European Union, the United Kingdom, Canada and other countries, and the holder of emergency-use authorisations or equivalents in the United States (jointly with Pfizer) and other countries. Pfizer and BioNTech also are pursuing regulatory approvals for this age group in other countries where emergency-use authorisations or equivalents have been granted.
Related
#HumanRightsMonth: NIH's potential funding cut threatens South African medical research 19 Mar 2025 Viatris fined in Morocco over merger notification, sources say 22 Nov 2024 African drug supply chain gets smarter with IBM-powered AI platform 14 Nov 2024 Leadership shakeup at Moderna ahead of major vaccine rollouts 8 Nov 2024 Legal and regulatory insights into South Africa’s growing medical cannabis market 2 Oct 2024 Study revolutionises global TB treatment 28 Aug 2024






































