
Subscribe & Follow
#AfricaMonth
Jobs
- Staff Nurse - Frail Care Strand
- Staff Nurse - Psychiatric Durban
- Account Manager Western Cape, Gauteng
- Sales Manager Sandton
- Clinical Specialist Cape Town
In the news
Cipla voluntarily recalls batches of Coryx throat spray
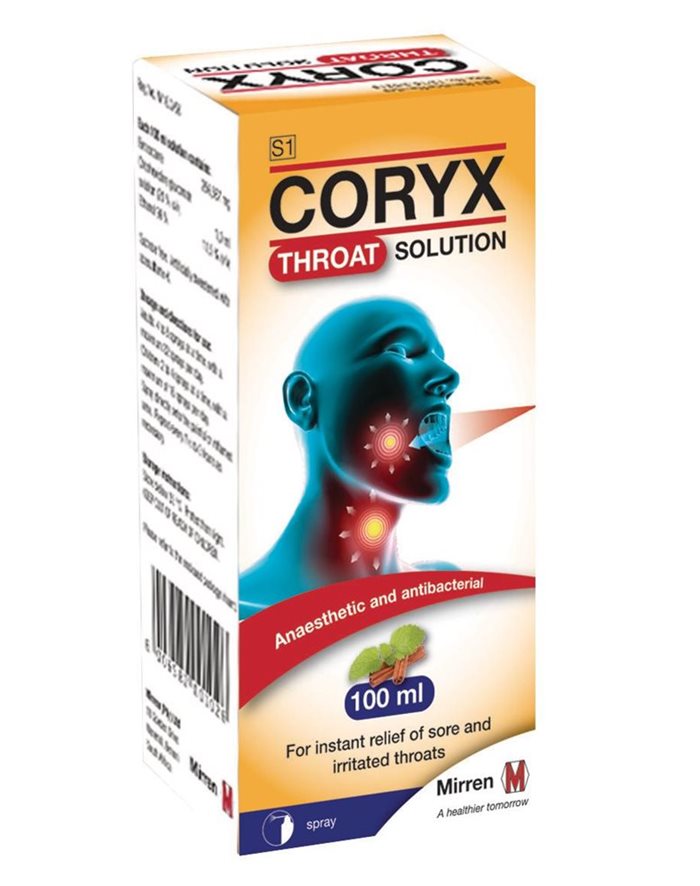
The reason for the Class I Type A product recall is due to the possibility of the spray nozzle detaching from the spray mechanism during use, resulting in the spray nozzle being ingested or getting stuck in the throat after detaching.
The batches being recalled are: ZB000156, ZB000157, ZB000158, ZB000159, ZB000160, as well as ZB100105, ZB100107, ZB100172 and ZB100173.
People who purchased any of these batches of Coryx throat spray are requested to return it to their pharmacy, and they will be refunded the purchase price.
The chief executive officer of Cipla South Africa, Paul Miller, said: “The safety and wellbeing of our patients is our utmost priority. Therefore, after liaising with SAHPRA, it was deemed necessary to recall specific batches of this product from the market.
"We are working closely with spray-mechanism manufacturers to ensure there is no margin for error in future.”
Related
Voluntary product recall: Heartland Foods cereal products 24 Mar 2025 Chronic medicine delivery reaches major milestone 11 Oct 2024 Unpacking the Sol de Janeiro fragrance fiasco - what is the brand's legal recourse? 11 Oct 2024 Product recall: Top Score Instant Porridge 1 Oct 2024 Woolies recalls Country Road Two-Tone Demm mugs 28 May 2024 Breathing new life into recycled asthma inhalers 21 May 2024








